Avidin Biotin Methods
Avidin is a basic glycoprotein (MW 68 Kd) which has a high affinity for the small (MW 24Kd) water soluble vitamin biotin. Biotin can be conjugated to a variety of biological molecules, including antibodies, and many biotin molecules can be attached to a single molecule of protein. The biotinylated protein can thus bind to more than one molecule of avidin.
However, avidin has two distinct disadvantages when used in immunocytochemical detection systems. It has a high isoelectric point of approximately 10 and is therefore positively charged at neutral pH. Consequently it may bind non specifically to negatively charged structures such as the nucleus. The second disadvantage is that avidin is a glycoprotein and reacts with molecules such as lectins via the carbohydrate moiety.
These two problems are overcome with the substitution of streptavidin for avidin. Streptavidin is a protein (MW 60 Kd) isolated from the bacterium Streptomyces avidinii , and like avidin, has four high affinity binding sites for biotin. Streptavidin has an isoelectric point close to neutral pH and therefore possess few strongly charged groups at the near neutral pH used in immunocytochemical detection systems. Furthermore streptavidin is not a glycoprotein and therefore does not bind to lectins. The physical properties of streptavidin therefore make this protein much more desirable for use in immunocytochemical detection systems than avidin.
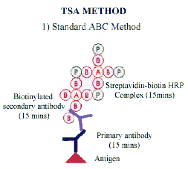
The most sophisticated and sensitive extension of the streptavidin biotin technique employs pre-formed complexes. Streptavidin and biotinylated horse-radish peroxidase are simply mixed at appropriate concentrations for at least 30 minutes at room temperature for the complex to form. The pre-formed complex is then attached to the biotinylated antibody. Careful stoichiometric control ensures that some binding sites remain free to bind with biotinylated antibody. This allows the pre-formed complex to bind and provides a very high signal at the antigen binding site.
Signal Amplification
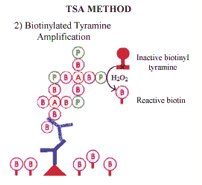
The technique requires appropriate pre-treatment procedures and optimal antigen retrieval, and follows a conventional ABC method. After the initial StrABC/HRP layer the biotinylated tyramide step is performed. |
|
|
|
||
|
|
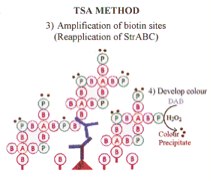
Horse-radish peroxidase (HRP) in the presence of hydrogen peroxide catalyses the formation of reactive biotinylated tyramide. This reactive biotinylated tyramide covalently binds to proteins via electron rich moieties such as the amino acids tryptophan and tyrosine, resulting in the deposition of biotin at the reaction site. Biotin deposition is restricted to the reaction site since horseradish peroxidase (in the form of StrABC /HRP) is only present at the antigenic site. Subsequent application of labeled streptavidin binds to the newly deposited biotin. |
|
||
The streptavidin may be labeled with enzyme (eg. HRP) or with appropriate fluorochromes. If enzyme labeled streptavidin is used the reaction can be visualized with the desired chromogen (eg diaminobenzidene). This technique has been used for both immunocytochemistry and for the detection of probes for in situ hybridization, and represents a significant advance in antigen detection. |
|
|
Advantages of biotinylated tyramide amplification
- Permits the use of greatly diluted primary antibodies.
- Enables shorter incubation periods.
- Provides amplification of weak signals
- Enables the demonstration of antibodies previously unreactive in paraffin sections.
- Increased number of steps
- Maybe prone to high background levels - especially in tissues rich in endogenous biotin.
- Expense.